Bringing Cell Therapies Closer to Patients with Innovative Manufacturing.
By Sofia Prado-Irwin, PhD

Lars Peeck, PhD is the Chief Business Officer at Astraveus, whose flagship product—the Lakhesys™ Benchtop Cell Factory™—is revolutionizing Cell and Gene Therapy (CGT) manufacturing. A distinguished leader in the life sciences business for over a decade, he has led global teams, launched dozens of bioprocess manufacturing products, and shaped strategy across multiple companies and portfolios. Orange County Bio spoke with Peeck about the growing impact of novel technologies in CGT manufacturing.
The Interview
Orange County Bio (OCB): What current gaps in cell therapy manufacturing inspired your company’s innovation, and when did you realize this technology needed to exist?
Lars Peeck (LP): Cell therapy manufacturing today is not at all industrialized. CGT relies on complex and often disjointed manufacturing processes, which makes therapies extremely expensive and often inaccessible for many patients. In short, the economics of the current system are broken.
Astraveus was founded to solve this problem by drastically simplifying cell therapy manufacturing. At Astraveus, we are developing a completely new manufacturing platform, the Lakhesys™ Benchtop Cell Factory™. The instrument is based on microfluidics, and leverages three core principles: miniaturization, parallelization and automation. With Lakhesys™, we can substantially reduce the cost of autologous CAR T-cell therapy manufacturing by at least a factor of five. The biggest motivation for us is to move the needle in terms of patient access and cost of manufacturing. I think there’s huge potential to do better.
OCB: When did you realize that this technology needed to exist?
LP: Shortly after I joined the company, I attended the American Society of Hematology conference where I saw clinical data presented on CAR T used against solid and liquid tumors as well as autoimmune diseases. I was deeply impressed by the huge therapeutic potential these data showed. But at the same time it’s so expensive to make these therapies that for some countries and health systems it’s just impossible to afford. That blew my mind.
OCB: What impact do you hope your work will have on both the industry and on individual patients and communities?
Our technology is a game changer in bringing therapies closer to patients and making them more affordable and accessible.
LP: Our technology is a game changer in bringing therapies closer to patients and making them more affordable and accessible. The platform is entirely automated and self-contained, making it suitable for use in lower-classification cleanrooms and enabling decentralized manufacturing. It supports short processing, reducing vein-to-vein time. And it substantially reduces the manufacturing costs of CAR T-cell therapy, making it more affordable and more accessible to patients.
OCB: What are some of the key scientific or engineering principles behind this novel technology?
LP: The core enabling technology is microfluidics, which offers a gentle environment that preserves cell health throughout the manufacturing process. The microfluidic scale enhances the efficiency of biomolecule transfer and gas exchange, significantly reducing the need for expensive reagents such as viral vectors—ultimately lowering the cost per dose. Another major factor in reducing both costs and footprint infrastructure is the parallelization of bioprocessing units, scaling out the simultaneous production of up to 10 patient doses within a single fully closed system.
We are delivering a fully automated system with integrated onboard analytical capabilities. For example, we’re incorporating an onboard and real-time fluorescence image-based analysis to reduce or replace offline flow cytometry. It also enables brightfield monitoring of cells during the process, thereby establishing advanced process analytical technology (PAT) approaches in cell therapy manufacturing.
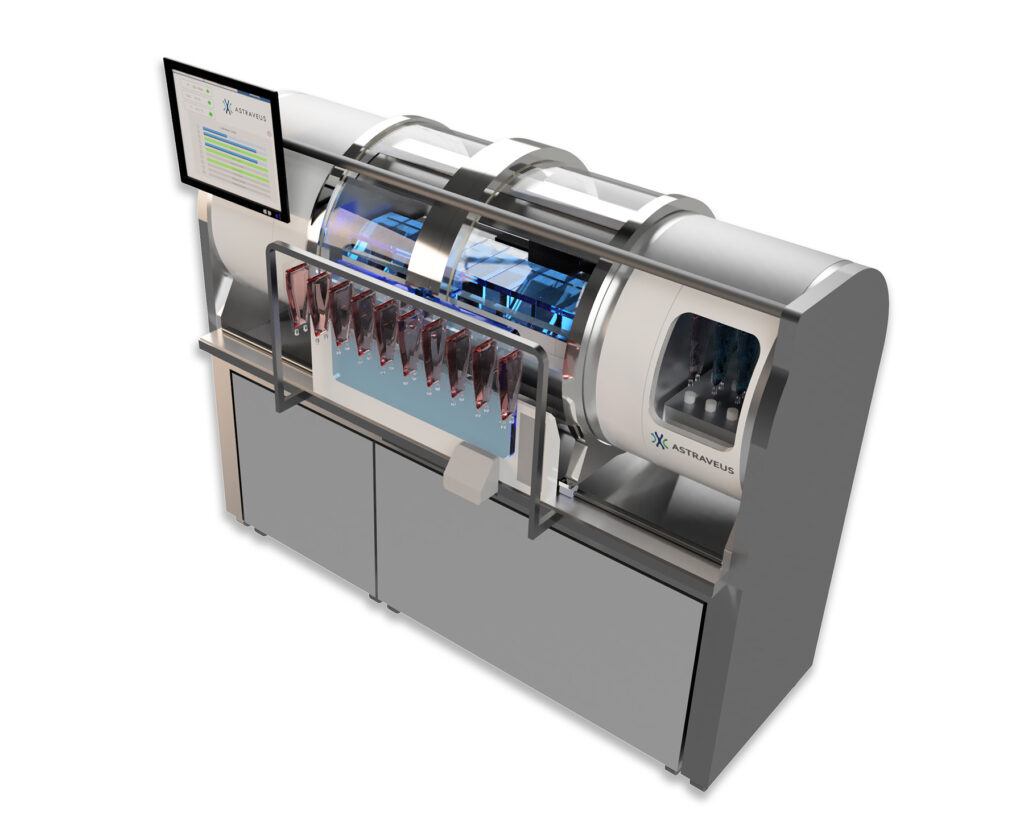
The Lakhesys™ Bench top Cell Factory™ integrates hardware, software & consumables from research to commercialization.
OCB: What are the key challenges and milestones you’ve faced in the development process?
LP: In 2022, the team built the first functional prototype of a system that can perform end-to-end cell therapy manufacturing on a microfluidic chip, which allowed us to perform a first on-chip end-to-end CAR T process at the end of 2024. That was a significant technical milestone.
In terms of challenges, our platform includes a lot of different technological foundations: microfluidics, mechanical and electrical engineering, digital tools and automation, as well as cell biology and surface chemistry. Bringing all that cutting-edge know-how together in a startup company that has limited resources is challenging. But we’ve been successful in building critical mass in all these verticals now, and our teams are collaborating very effectively to maximize the value of their complementary skills.
OCB: How does your platform ensure scalability and consistency while maintaining quality and regulatory compliance?
LP: In terms of consistency, parallelization and end-to-end process execution in one system eliminates certain external variabilities. All ten bioprocessing units share the same environment, software, and culture conditions, making it easier to control across batches.
… not having to switch technologies between preclinical, clinical, and commercial stages removes another source of variability.
Regarding scalability, not having to switch technologies between preclinical, clinical, and commercial stages removes another source of variability.
In product development, we follow design control principles and a framework that entails stringent specifications, testing, and validation to ensure repeatable product quality.
OCB: How do you envision your technology evolving over the next 10 years?
LP: Our technology provides the opportunity to integrate a lot of other features to streamline process development and simplify manufacturing. One example is analytical capabilities. We’ve started with bright field cell imaging and image-based cytometry, but could we also include functional testing in the future?
Non-viral transfection technologies is another example to consider. Whether it’s with electroporation, mechanoporation, other transport vehicles of genetic material such as LNPs, the prospects are certainly less than ten years away.
Right now, we are focused on autologous CAR T processing, but many other cell therapy modalities would benefit from our technology, and we would like to look into these applications in the future.
In Reflection
Innovation in cell and gene therapy (CGT) manufacturing is critical to expanding patient access to life-saving treatments. The team at Astraveus continues to demonstrate how to harness the untapped potential of innovative technologies, ensuring they are fully leveraged to address the evolving needs of the CGT industry. With 15 years of product development experience in the bioprocess industry, Lars Peeck, PhD emphasizes the combination of disruptive technology and robust process control to achieve tangible real-world impact. The work of Astraveus exemplifies how novel manufacturing technologies can accelerate process development, reduce costs, and ultimately make CGT therapies more accessible to patients.
